MonQual QMS comes with pre-built, DMS and LMS systems. But do you know it offers integration with various stand-alone systems too? You can integrate your LIMS (Laboratory Information Management System), MES (Manufacturing Execution System), SAP, WMS (Warehouse Management System), etc. This applies for both legacy and modern applications.
MonQual is everything your quality team dreams of. It combines quality with compliance management, business intelligent tools, and business productivity. A built-in DMS and LMS services along with automated workflows takes care of your documents and regulatory standards. Isn’t it, Quality Harmonized?
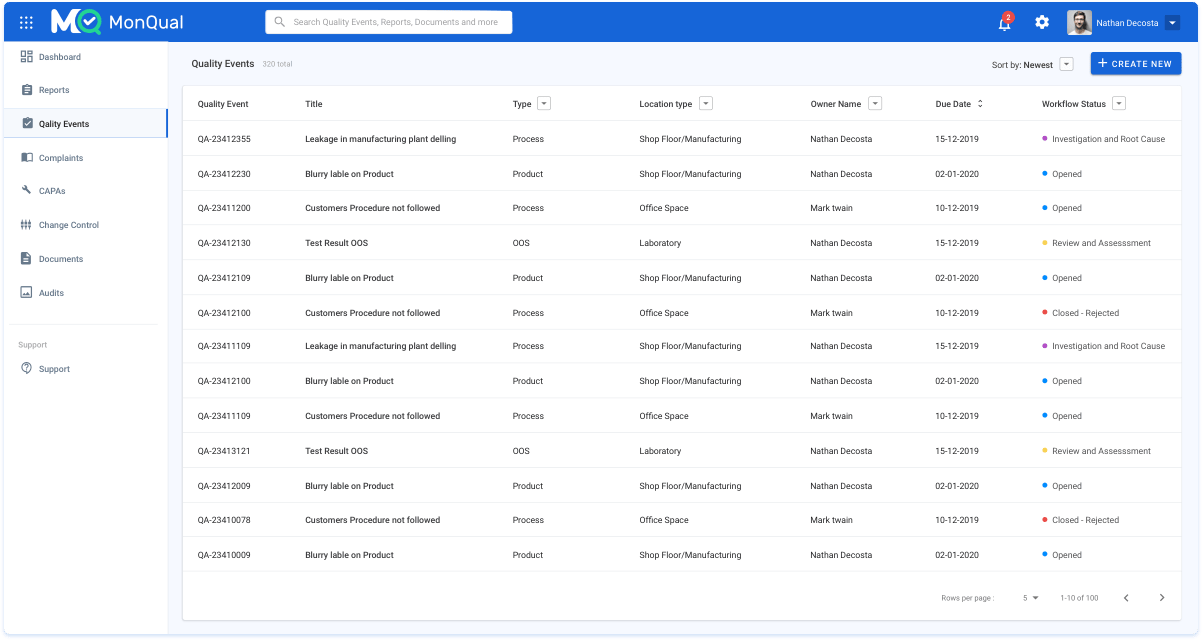
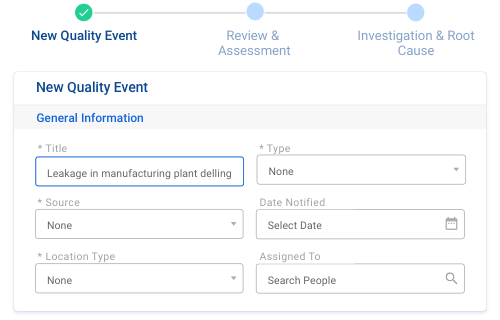
Quality deviations are a part of process; however, we can stop avoid them in the future. MonQual offers processes that can identify deviations at an early stage, tracks them and runs a root cause analysis and finally, records and documents them to avoid future recurrence.
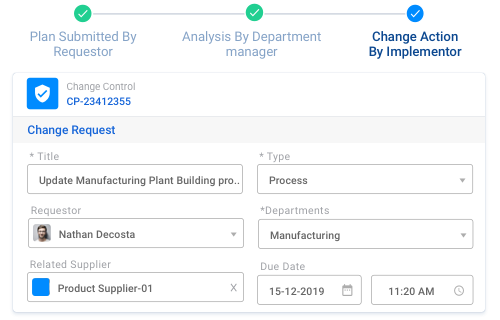
Ensure continuous improvement effectively without unintended consequences. It is possible with our Change Control System. Automated workflows with access control ensures proposal reviews and changes that may impact controlled states are validated.
Training is crucial in pharma and life science industry; it helps organizations enhance their knowledge. MonQual offers integrated LMS that complies with as per 21 CFR Part 11, EU Annex 11, GAMP, MHRA, GMP or cGMP, ISO and other regulatory standards. The users can access or conduct different types of trainings based on their job profiles.
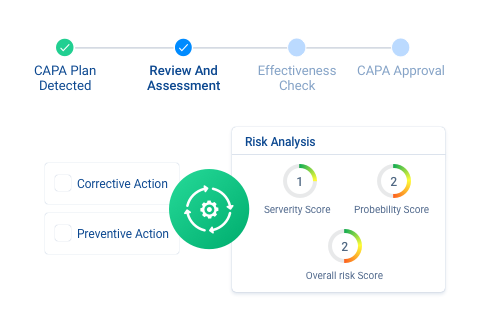
MonQual’s integrated with an automated CAPA management. Track any deviations from expected quality and reduce risks of future recurrences. In addition, system also generates automated emails and notifications for approaching due dates or renewals.
Data and document never stop. DMS (Document Management System) integration with MonQual QMS helps organize documents, eliminate redundancy, aid meet regulatory standards and improve audit readiness. The DMS has been specifically designed to be compliant with FDA 21 CFR Part 11 including electronic signatures, password authentication and immutable Audit Trails.
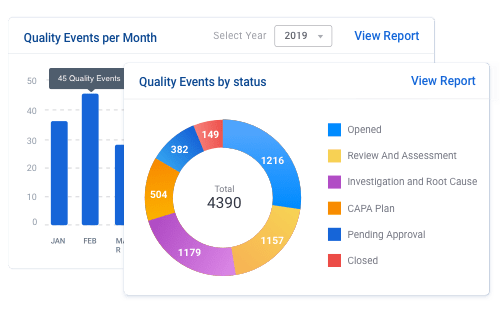
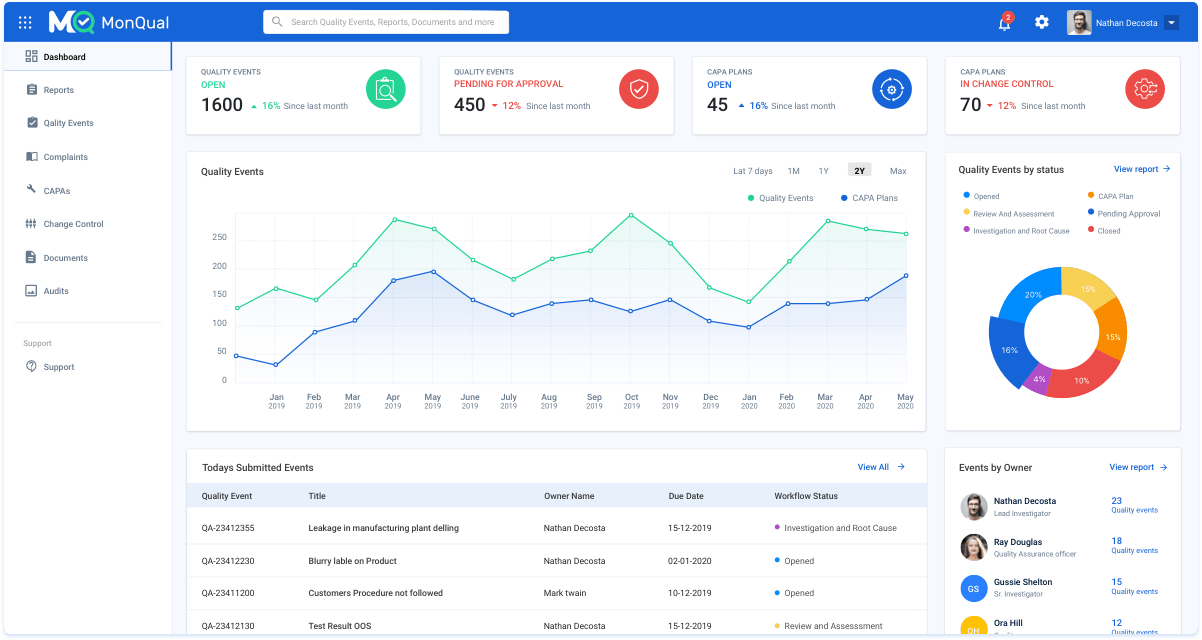
With MonQual’s reporting analytics uses Power BI to bring your data to life. Connect to data from cloud or on-premise, structured or unstructured. Get insights through interactive dashboards, monitor quality trends and turn them into actionable insights. Shift from problem detection to problem prevention with live dashboards.
Manage audit processes with a reliable centralized system that can be assessed by assigned individuals only. Automate audit processes to improve quality, meet industry specific regulations and get compliance faster.

Heavy upfront investments, ease of use, make legacy system migration difficult. However, these systems like LIMS (Laboratory Information Management System), MES (Manufacturing Execution System), SAP, Warehouse Management System, etc. are business strategic so, MonQual offers, API based integration to these systems. This helps integrate quality into various peripherals of operations.
Microsoft 365 has become synonymous with business productivity and collaboration. Get most out of the experience and leverage the combined power of QMS, and business collaborative platform. It’s better together.

MonQual offers affordable Quality Management Service, is perfectly harmonized and easy to get started. In addition, we at MonQual offer the entire stack of service from design to support.